Description
What is pH?
On a logarithmic scale, pH (potential of hydrogen) represents the acidity or alkalinity of a water-based solution. On this scale, 7 represents neutrality, lower values indicate acidity, and higher values indicate alkalinity.
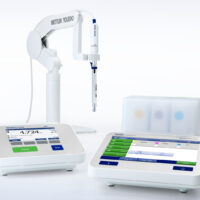
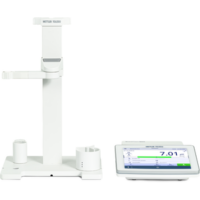
What are pH probes and how do they work?
A pH probe allows a user to determine the alkalinity or acidity of a solution. pH probes work by measuring a solution’s hydrogen-ion activity, which is detected by a sensitive glass membrane at the end of the probe. For accurate measurements, it’s important to regularly calibrate the pH probe and maintain it according to the manufacturer’s instructions.
When exposed to an aqueous solution, the exterior surface of the glass membrane develops a gel layer. Because the pH probe is filled with an aqueous electrolyte solution, a similar gel layer forms on the inner side of the glass membrane. Depending on the pH value, the H+ ions in and around the gel layer can diffuse into or out of this layer. As a result, the solution’s H+ ion concentration is determined.
If the solution is alkaline, H+ ions diffuse out of the layer and a negative charge forms on the membrane’s outer surface. If the solution is acidic, H+ ions diffuse into the layer and a positive charge forms on the membrane’s outer surface. Because the pH probe contains an internal buffer with a constant pH, the potential on the inner surface of the membrane remains constant throughout the measurement. As a result, the pH probe potential is the difference between the inner and outer charges of the membrane.